Containment of APIs released through component washing
Personnel and environmental safety are critical for pharmaceutical companies that manufacture drugs with hazardous or potentially hazardous Active Pharmaceutical Ingredients (APIs). APIs are a normal byproduct of pharmaceutical production, research and development that can be released into the loading and service area via the component cleaning/washing process.
Pharmaceutical companies that manufacture drugs with hazardous or potentially hazardous APIs therefore need a solution that works with cGMP washers to ensure the safety of personnel and the environment during the cleaning of drug manufacturing equipment and component parts.
The Solution
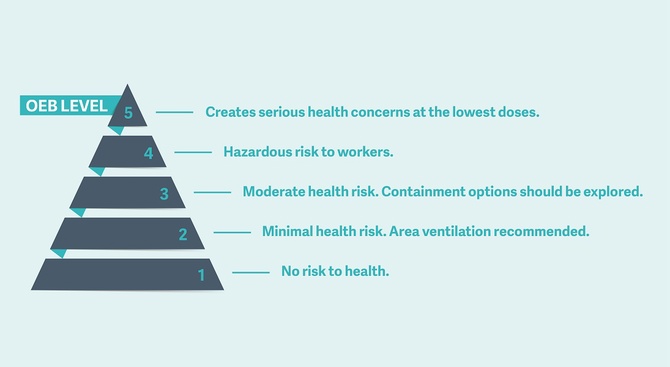
The Getinge Air Exhaust Unit is an optional, standalone solution that captures potent and highly potent APIs and prevents their release into the production, service, and loading areas. Mitigation of trace API residuals emitted after a component washing cycle through the use of an Air Exhaust Unit can reduce worker exposure near the washing process. The system uses a highly effective bag-in, bag-out HEPA filtration system to capture the residual HPAPIs and APIs removed during the equipment cleaning process, which prevents the release into the service and other adjacent areas. In addition, the Air Exhaust Unit provides level 5 OEB containment for the highest level of personal safety from API particulates.